What to Expect
Follow along with our step-by-step guide to the MitraClip™ transcatheter edge-to-edge repair (TEER) procedure for mitral valve repair.
The MitraClip Therapy Procedure
MitraClip TEER is a minimally invasive procedure
The MitraClip Therapy procedure is performed in a hospital by a specialized care team, but is not surgery. The doctor repairs the mitral valve through a small puncture in your groin, rather than an open-chest incision.
The procedure is performed under general anesthesia and typically lasts 1 to 3 hours.1 People typically return home after 1 day in the hospital.1
The following steps provide a general overview of the MitraClip Therapy procedure—your experience may be different. The doctor can explain the procedure to you, provide specific details, and answer questions you may have.

Here’s what happens:
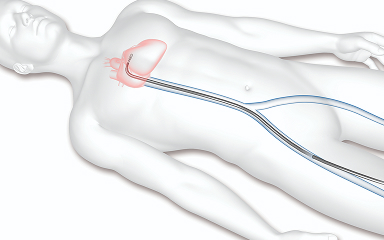
Access through a vein
The doctor will make a small puncture in the groin to access a vein and inserts a catheter to reach the heart.
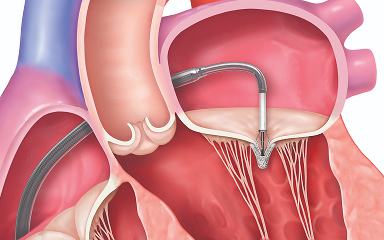
Placing the MitraClip implant
The implant is guided to the heart and placed in the mitral valve. One or more implants may be used to repair the leak.
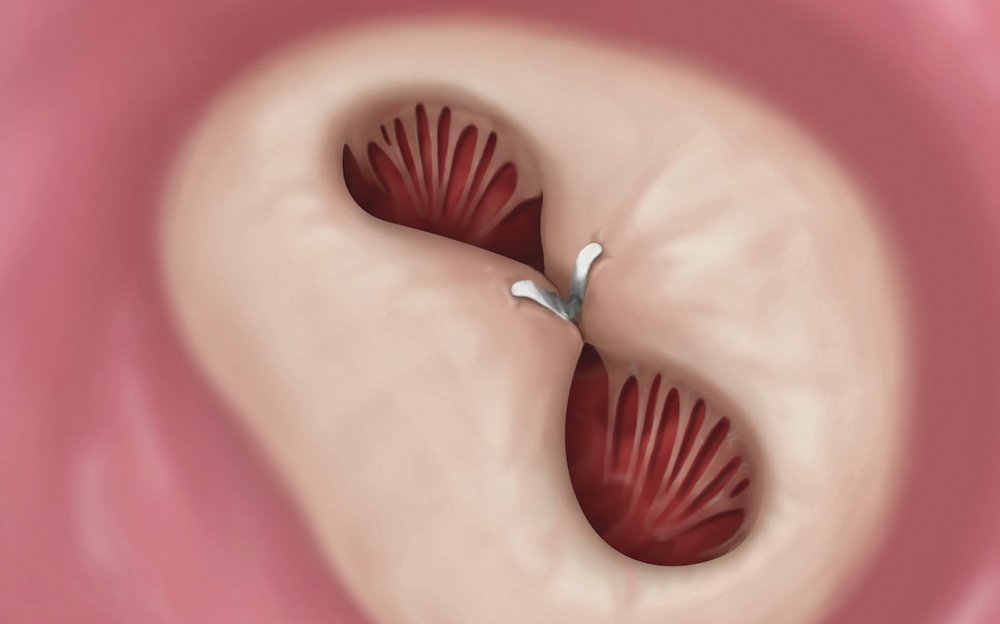
Finishing the procedure
The catheter is removed and the MitraClip implant becomes a permanent part of the heart. Once in place, the implant helps the valve close more tightly. When the sides of the valve open, blood flows around the implant, into the ventricle.
Recovery
The path to healing and feeling better
Most patients go home the next day.1 But before you leave, the doctor will discuss next steps with you.
They will give you specific instructions to help you with your recovery. It is important to carefully follow the doctor’s directions, especially if you need to take any medications.
The procedure can provide immediate reduction of mitral regurgitation. You should experience relief from your symptoms soon after your procedure. However, it's still normal to need a bit of rest as your body heals. You should be back to light activities after 2 weeks.1
Important recovery tips:
- You will need to keep the small incision area in your groin dry for the first 24 hours. This is where the doctor accessed your vein for the procedure.
- You are likely able to take a shower, but avoid soaking the access site.
- Avoid perfumes, lotions, etc near the access site.
- If bruising around the access site suddenly gets bigger or harder, call your doctor immediately.
- If any bleeding occurs, or if symptoms worsen, you may need to go to the emergency department.
Following Up
Checking in after your heart procedure
Regular check-ups with your doctor are very important. You will be released to the care of your cardiologist or family doctor, and you may be asked to return for follow-up visits per your doctor’s directions. It’s important that you reach out to your doctor whenever you have questions or concerns about your health.

Your Abbott Implant Card
As you leave the hospital, you will receive an implant card with information about your MitraClip Therapy. Please share your implant card with your healthcare team and before any medical, dental, or magnetic resonance imaging (MRI) procedures.
Is MitraClip Therapy right for me?
Use our quick questionnaire to see if you might be a candidate for MitraClip Therapy.

Ready to discuss with your doctor?
If your mitral regurgitation symptoms are interrupting your daily life, download this discussion guide to prepare for your next doctor’s appointment.




